Can our chromatography columns be indestructible and serve forever? We all know this is impossible, but there are ways to prevent columns from being too short-lived.
Do we feel we are spending all of our lab funds on columns? Do we wonder why chromatography manufacturers can’t produce columns that last longer? If properly protected during use, the cost of purchasing a column is a small fraction of the total cost of chromatography analysis. The normal life of a column is about 500-1000 needles, and if we take this figure, the cost of the column is only about 1-2% of the total cost of analysis. So even if we feel that the unit cost of a column is high and we want to try to add extra protection to increase its lifetime, it does not save much for the overall analysis. I think if a column can have 500 injections, it can be considered to have fulfilled its mission. What is a reasonable expectation of column life in general? And what protection measures are available to extend column life?
What is a reasonable expectation for the life of a column?
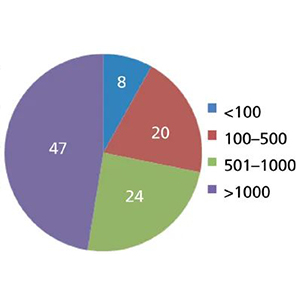
First, look at the findings of another chromatographer, Ron Majors. In his published article, he researched the life of liquid chromatographic columns. Figure 1 shows the data from the 2011 study in his article, and we can see that nearly 3/4 of the respondents could get at least 500 injections (24% + 47% = 71%). Nearly half could get at least 1000 injections (47%); this is surprising because the study included various column types, not just the more commonly used and durable 150 mm × 4.6 mm size reversed-phase columns with a particle size of 5 μm.
Figure 1 End-of-life sample volume per column in 2011
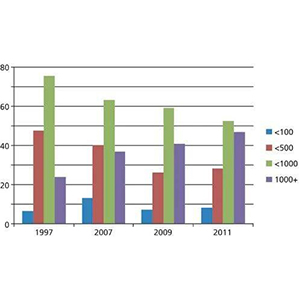
Several points are worth mentioning in the changes from 1997 to 2011 (Figure 2).
(1) The proportion of samples injected with less than 1000 needles (green) decreased yearly from 76% in 1997 to 53% in 2011.
(2) The proportion of samples with an intake of more than 1000 needles (purple) has increased yearly.
(3) the proportion of sampling volume of 500 needles or less (brown) decreased from about 50% in 1997 to about 25% in 2011
(4) The bad results of the sample volume of 100 needles or less (blue) have remained at about 10% for many years.
Figure 2 Comparison of sample volume before the end of column life from 1997 to 2011
I have made the following bold speculations about these results: First of all, I do not think that the increase in column life is mainly due to the technological progress of chromatography manufacturers’ production because after 1997 (the beginning of this paper’s research), the technology of loading columns has been considered very mature. So I think the main reason for the increase in lifetime is that laboratory personnel have been better educated and trained in using columns, meaning that laboratory personnel understand how to pre-treat samples and protect the columns. And I suspect that the main reason for those with less than 100 injections is improper handling, not a column problem. This article will provide some suggestions on how to protect the column.
Never sample “garbage.”
I often joke that the way to protect a column from getting the most life out of it is not to open the column. Once you start using the column, the column’s life decays until it breaks down. The two main factors that affect the life of a column are impurities and particles in the sample, so if we can remove impurities and particles from the sample before feeding it, the column’s life will be extended. The pretreatment methods include solid phase extraction (SPE), liquid-liquid extraction and other filtration methods. If the contaminants are substantially reduced before the sample enters the column, the column’s life will be extended, but we must also consider whether the time and expense spent on pretreatment will be justified. Suppose the column currently in use is already at 500 injections, and the increased time and cost of using pretreatment is such that the column life needs to be increased from 500 injections to 2500 injections to make it worthwhile. In that case, one must consider whether pretreatment is economically beneficial. However, if plasma samples are fed without pretreatment, the column may be ruined in less than 10 injections, and this is when pretreatment is very necessary. But in addition to the economic benefits, a comparison must be made of chromatographic data’s advantages and disadvantages without pretreatment. In our laboratory, these pretreatments often allow us to collect valid data even at lower concentrations and also allow us to get more consistent results.
It is recommended that all samples be filtered or centrifuged before injection, with or without pretreatments. For example, when the sample is filtered through a membrane to remove particles and thus reduce clogging of the column’s frit, the pore size of the membrane must be smaller than the column’s frit pore size. For columns with particles larger than 3 μm, a 2 μm frit is used; for columns with 3 μm particles, a 0.5 μm frit is used; and for columns with particles smaller than 3 μm, a 0.2 μm frit is used. Filter membranes with large and small pores. Most filter membranes are pre-encapsulated and can be attached directly to a syringe for filtration or used as a filter plate for a 96-well plate.
Although the use of membranes is effective, it is recommended that the following four points be considered first.
(1) Filter membranes are not cheap, and while the unit cost of each one is not very expensive, it does not add up to a cheap cost.
(2) Filtration takes extra time, which is slow with 0.5 μm membranes and even slower with 0.2 μm membranes.
(3) Strictly speaking, once a sample is filtered, it has to be filtered for other samples, whether filtration is necessary or not, to avoid unnecessary variables interfering with the experimental data.
(4) Once filtration is used, the filtration procedure must also be validated when validating the method development. Since the filtration process tends to lose samples, we need to determine if there are inconsistencies in the degree of loss of different components, especially at high and low concentrations.
Since using membranes is time-consuming and costly, each laboratory must assess whether it is worthwhile. However, there is no denying that using membrane filtration of samples will, in most cases, increase the column’s life.
Another way to replace or supplement membrane filtration is to centrifuge the sample before injection. This is a simple and economical method. After centrifugation, some larger particles that tend to clog the frit settle at the bottom of the tube and can then be easily sampled for analysis. We have used this method for many years for protein samples and have had a few problems with particles clogging the column. I have had the experience of using UHPLC, where the sample was filtered through a 0.2 μm membrane but was still cloudy, and the column was clogged with this sample. Still, the column clogging problem was solved once the cloudy sample was centrifuged and the sample was no longer cloudy.
Using HPLC inline filters
I am convinced that an inline filter is the most cost-effective component of an HPLC or UHPLC instrument. Inline filters are placed between the autosampler and the column (or HPLC guard column). Commonly used inline filters are stainless steel inline filters with replaceable sieves (0.5 μm or 0.2 μm) and disposable HPLC inline filters made of PEEK. It is generally necessary to choose a frit with a smaller pore size than the column frit to effectively intercept particles that may clog the column frit. Once you notice that the instrument’s pressure increases, the inline filter plate may start to clog, and we must renew it before it is completely clogged.
The HPLC inline filter functions like a filter membrane but does not require additional work. After treating the sample with a centrifuge, a few particles may still enter the instrument. The inline filter, located in front of the column, will intercept the residual particles. In addition, the inline filter is easy to change, typically taking only 5 minutes to change, and is ready for use without spending time on readjustment or calibration. Another advantage is that the inline filter can also filter out the particles generated by the ageing of some parts of the instrument. I strongly advocate that every liquid phase instrument be equipped with an inline filter; even if a guard column is used, I recommend that an inline filter be installed in front of the guard column.
Using HPLC guard columns
A HPLC guard column can be described as a short column mounted in front of a chromatographic column. It is usually 1-2 cm long, packed with packing that matches the column packing used and using a frit with an aperture no larger than the aperture of the frit used in the column. The guard column has two functions:
(1) it blocks particles that may clog the column;
(2) because the guard column matches the column packing, any chemical impurities that may interact irreversibly with the column packing and not produce a peak will be captured by the guard column first.
Using a guard column can extend the column’s life. However, before considering whether to use a guard column, it is recommended to evaluate the following points.
(1) Guard columns are not cheap either, so consider whether the increased column life can compensate for the cost of purchasing a guard column.
(2) How long does the protective column last?
It is important to note when the protective column core needs to be changed and not contaminate the column by replacing it too late.
(3) How to flush the protective column?
The flushing requires separate flushing of the protective column and the chromatographic column to avoid flushing impurities accumulated in the protective column into the column.
(4) Do not expect that adding a 1-2 cm protective column will increase the column efficiency. Although installing a guard column is similar to increasing the column length, using a guard column also adds two joints and connection lines, reducing the instrument system’s column efficiency. Hence, it is good to maintain the column efficiency without the guard column.
Using a guard column will certainly extend the column’s life, but it is recommended that each lab evaluate the need for a guard column in terms of cost-effectiveness and workload. Even if a guard column is used, I recommend installing an inline filter between the guard column and the autosampler. After all, replacing the inline filter is cheaper than replacing the guard column and increases the life of both the guard column and the chromatographic column.
In summary: Although columns cannot last forever, there are some simple methods we can adopt to extend the life of a column. First, even if the sample requires pretreatment, it is recommended that the sample be further processed by filtration or centrifugation as a final step; second, use an inline filter or a guard column to protect the column by intercepting particles in the sample or from the instrument itself; third, evaluate whether a guard column is worth using based on your analytes; and finally, remember to use a strong solvent at the end of each analysis to rinse out any impurities retained by the column. Finally, remember to use a strong solvent to rinse out impurities retained by the column after each analysis.
References
(1) John W. Dolan, LCGC North America, 32 (12), 916 – 920 (2014)
(2) R.E. Majors, LCGC North America, 30(1), 20-34 (2012).
Post time: Sep-09-2022