What are the reverse phase column and normal phase column?
“Inverse” and “positive phase liquid chromatography (HPLC) are the concept of” early put forward the concept of the bonded phase chromatographic column has not yet appeared. The stationary phase is coated on the surface of the carrier. It is easily lost, so scientists’ reasonable Suggestions are provided for the use of the mobile phase: the polarity of the mobile phase and stationary liquid polarity should have a bigger difference to reduce fixed fluid loss. Liquid chromatography, when the polarity of the stationary phase is weaker than that of the mobile phase, is called reverse-phase chromatography, and liquid chromatography, when the polarity of the stationary phase is stronger than that of the mobile phase, is called normal-phase chromatography. Although CHROMATographic bond columns have become mainstream, this concept is of great significance in developing chromatographic methods and predicting peak order.
From the above introduction, it can be seen that the specific chromatography method and whether the column belongs to the normal phase or the reverse phase depend not only on the stationary phase but also on the polarity of the mobile phase. C18 (Silica gel bonded to octadecylsilane), C8 (Silica-bonded octylsilane), PH (silica gel bonded phenyl silane), and other columns are standard reverse-phase columns because of the very low polarity of the stationary phase, which is lower than that of any known mobile phase.
Silica gel and NH2 silica gel bonded aminopropyl silane has high polarity and are mainly used to separate compounds with polar groups. The polarity of the mobile phase is usually lower than that of these stationary phases, so they are standard normal-phase columns. The polarity of CN (silica gel bonded nitrile propyl) is moderate. When the polarity of the mobile phase exceeds CN, it belongs to the reverse phase column. Otherwise, it is a positive phase column.
How does column specification affect the analytical results?
The inner diameter of the column determines the sample load, which is proportional to the square of the inner diameter. The column length is directly proportional to the number of plates and the column pressure. Particle size affects the vortex diffusion phase. The smaller the particle size, the smaller the vortex diffusion phase and the higher the column efficiency. The particle size is approximately inversely proportional to the column efficiency. The smaller the particle size, the higher the pressure, which is inversely proportional to the square of the particle size. Packing aperture molecular weight has limitations for the analysis object. When the aperture size for the analyte is above 5 times, analytes can flow smoothly through the pore. The pore size in a 60 ~ 120 Å chromatographic column is suitable for the analysis of the relative molecular weight is less than 10000, aperture for a 300 Å chromatographic column can meet the molecular weight in the analysis of macromolecular compounds of more than 10000.
How to improve the separation in liquid chromatography analysis?
The following formula is the separation degree calculation formula:
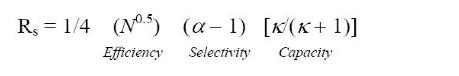
N: Efficiency reflects column performance. The higher the column efficiency, the better the separation. When the other conditions are constant, the separation degree increases by only 40% when the number of plates doubles. In operation, the number of trays can be increased in the following two ways to improve the separation degree: first, the use of long columns or double columns in series, but also make the separation time greatly prolonged; Second, columns with fine particle size fillers are used, but this requires liquid chromatography systems that are resistant to higher pressures. The latter is preferable.
Selectivity refers to the ability of a columnar mobile phase system to separate two compounds. The selectivity is mainly related to the composition of the stationary phase, mobile phase, column temperature and other factors. It is closely related to the retention value, among which the composition of the stationary and mobile phases greatly influences.
In one of the most common inverse models, for example, reversed-phase column (including the C18, C8, PH, etc.) and distribution are to keep the compound, the separation of different compounds based on their differences in bonding phase flow phase distribution coefficient, if two compounds solubility in water, in such aspects as the distribution coefficient of alkane – water system exists obvious difference, These compounds can usually be separated by reverse-phase columns; The PH column has special reservations for compounds with benzene rings.
In normal phase mode, the silica gel column, amine column, cyano column and compounds with polar groups have polar interactions, which are selective to the groups of compounds, and are often used for separating structural analogues and isomers. For the mobile phase, reducing the elution strength of the mobile phase usually increases the separation degree. For example, under reverse-phase conditions, the selectivity of acetonitrile and methanol is very different, which needs to be explored in practice. However, various solvent types bring us more possibilities to achieve separation.
k: With the capacity factor k, separation also increases. This effect is very obvious when the value of k is low. When the value of k is greater than 10, the effect of increasing the value of k on the degree of separation is no longer significant, which warns that it is meaningless to increase the value of k evil to increase the degree of separation. Increasing the bonding phase density can increase the k value. In addition, changing the bond group type can also change the k value. For example, in reverse-phase chromatography, the k value gradually increases with the increase of the bond carbon chain length.
What is capped? What is the meaning of the encapsulation?
The density of Si-OH on the surface of silica gel is 8 μmol/m2. Due to steric hindrance, the silylation bonding reaction can only cover 50% of the silica hydroxyl groups at most, and more than half of the silica hydroxyl groups are active. These silicon hydroxyl groups have polar and ion-exchange interactions with the polar groups of the compound, adding undesired forces to the compound’s retention and often affecting the peak shape. This effect can be reduced or eliminated by bonding an active silicon hydroxyl group with a short-chain chlorosilanes (e.g., trimethylchlorosilane), a procedure known as Endcap.
The significance of end sealing treatment: inhibited the specific adsorption, improved the symmetry of chromatographic peak, and improved the separation effect; To some extent, the surface of silica gel is masked, and its tolerance to an alkaline environment is enhanced. The steric hindrance covers the Si-O-Si bond formed by the bonding reaction, which enhances its tolerance to an acidic environment. It may affect the selectivity of polar samples.
Column bed collapse refers to the formation of visible gaps in the column bed at the column entrance after the column has been used for some time. The existence of this void increases the dead volume and results in a decrease in column efficiency. The causes of column bed collapse are as follows: first, the pressure of column filling is too low, the filling is not close, and after a period of use under high pressure, the gap begins to appear; Secondly, the operating pressure exceeds the pressure tolerance value of the column packing, resulting in the breakage of the packing particles and the formation of voids. Third, the mobile phase dissolves the filler, leading to the emergence of voids. Bond phase collapse refers to the fact that the bond phase cannot be fully extended in the mobile phase due to the large difference between the polarity of the mobile phase and the bond phase, so the bond phase becomes lodging and entangles together, such as standard C18 in the pure aqueous phase. Phase collapse may result in poor retention of compounds by the column.
Cause and elimination of elevated system pressure
The user can monitor the system’s pressure by observing the instrument’s system. If the pressure is high, do not immediately assume that the column pressure is high because the system pressure is usually composed of the pre-column pressure, column pressure, and flow cell pressure. At this time, the correct practice is: under the operating condition, measure the system pressure, and obtain p total; The column was unloaded, and the pressure before the column was measured under the same conditions to obtain p. Connect the outflow line of the pump with the flow pool by two ways. Under operating conditions, measure the pressure and get p(front + back). Calculate whether the pressure is coming from the front or back of the column.
|
The specific phenomenon |
Possible reasons for |
The solution |
Prevent problems from occurring |
|
High column front pressure (> 1MPa) |
inline filter clogging |
Clean or replace the frit in the in-line filter |
The most fundamental cause of pressure rise is the presence of solid impurities and strongly retained substances in the mobile phase or sample. To avoid the recurrence of the problem, the following two things should be done. 1. filtration of the mobile phase and sample solution must be in place. 2. install a guard column in front of the chromatographic column |
|
tubing jam |
Replace clogged tubing |
||
|
High post-column pressure (p > 1 MPa)
Gradual increase in column pressure |
Circulation tank contaminated or blocked |
Rinse the circulation cell with a variety of solvent systems of different polarity |
|
|
tubing jam |
Replace clogged tubing |
||
|
Particulate impurities accumulate in the frit or stigma |
Regeneration of the column with multiple solvent systems of different polarity; flushing of the column in the opposite direction; cleaning or replacement of the HPLC frit; replacement of contaminated packing in the column head |
||
|
Strong retentive material accumulates on the filler |
|
||
|
Sudden rise in column pressure |
The samples contained more strongly retained substances or were not filtered |
Continued effective pre-treatment of samples |
|
|
Buffer salting out |
Ensure buffer solution and organic mutual accommodation; transition from the organic phase to the mobile phase containing the buffer solution with a mixture of solvents containing higher levels of water |
The above scenario assumes that no guard column is fitted. If a guard column is fitted, the pressure of the protective column should be tested first when the pressure rises to confirm whether the problem is coming from the guard column.
Decline in column performance:
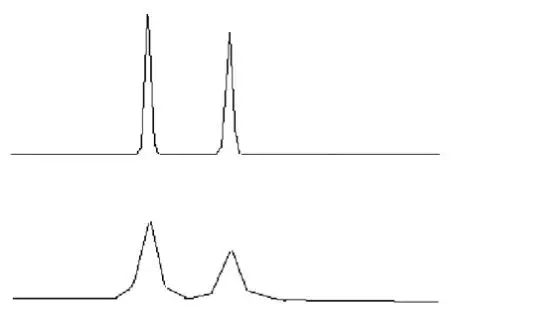
An important phenomenon in the degradation of the column performance is the significant increase in the regional width of the peaks with essentially no change in retention time (figure below).
When you experience a drop in column efficiency, please follow the table below to troubleshoot and resolve the problem.
|
The specific phenomenon |
Possible reasons for |
The solution |
Note |
|
Chromatographic peak area broadening |
Large dead volume outside the column |
Replace the connection tube with a smaller inner diameter and a shorter length; connect the connection tube tightly to the column; thin diameter column, use a smaller flow cell. |
To ensure that the column has a high column efficiency, the HPLC tube connections should be strictly controlled to ensure a small dead volume; to preserve the column performance for as long as possible, the purity and filtration of the mobile phase must be in place, a guard column should be installed, and the column should not be operated under extreme conditions. |
|
Column bed collapse |
Unable to recover |
||
|
Loss of packing by mobile phase and collapse of inlet column bed |
Partial recovery of column performance is possible by filling with the same type of packing. |
||
|
Regional broadening with peak trailing
Regional broadening accompanied by peak front extension |
Clogging of the frit or column head or adsorption of strongly retained compounds to the column head (accompanied by increased pressure) |
Clean or replace frit with strong eluting solvent; dig out contaminated packing and replace it with clean packing. |
|
|
Presence of specific adsorption (with trailing) |
Use low pH inhibition, end packing, and add competitor. |
||
|
The sample solvent is more eluting than the mobile phase |
Dissolve with a solvent with low elution capacity, preferably in the mobile phase |
||
|
sample overload |
Reduce sample concentration or volume |
Post time: Oct-21-2022






