
The application of the HPLC method in research is content determination. What issues must be noted when applying the HPLC method to content determination? What precautions must be taken to calculate the content of external standards?
In addition to the identification and related substances examination, the application of the HPLC method in chemical drugs is content determination. In addition to preparing the content uniformity, dissolution, and release test items, the HPLC method also plays an increasingly important role. Still, its essence and content determination are accurate quantitative determination methods. Therefore, the content determination also includes content uniformity, dissolution, release, and another quantitative testing. The HPLC method plays an increasingly important role.
1, Problems to be noted when applying the HPLC method to content determination When establishing a new way for content determination, the method establishment, as well as the methodological validation protocol, requires special consideration of the influence of several factors on the analytical results, as described below.
(1) The pH of the mobile phase buffer salt affects the retention behavior of the drug and can even significantly alter the retention behavior of some essential medicines. In Figure 1 below, when the pH of the buffer solution in the mobile phase is 3.0, the order of peaks is lidocaine, benzamide, and ketoprofen. When the pH of the buffer solution in the mobile phase is 10.0, the order of peaks for the three drugs is ketoprofen, benzamide, and lidocaine. 2 Even with gradient elution, the pH of the buffer solution in the mobile phase still affects the retention behavior of the drug.
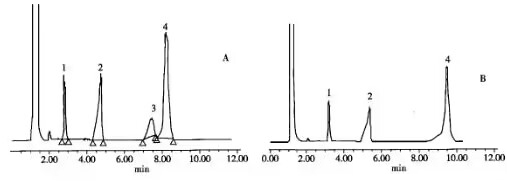
Figure 1 Effect of mobile phase pH on retention time
(2) Chromatographic columns differences in columns can also affect the retention behavior of drugs. Even for lc columns with the same length, inner diameter, and particle size, drugs’ retention time and peak shape can sometimes differ significantly due to differences like the packing material.
(3) The mobile phase composition was used as an example for determining the content of a multivitamin preparation containing four vitamins: niacinamide, vitamin B6, vitamin B2, and vitamin B1. As shown in Figure 2 when the relative ratio of sodium hexane sulfonate and sodium heptane sulfonate in the mobile phase was changed from 1:1 to 4:6, not only did the retention time and peak shape of each component change but even peak three and peak four did not achieve separation.
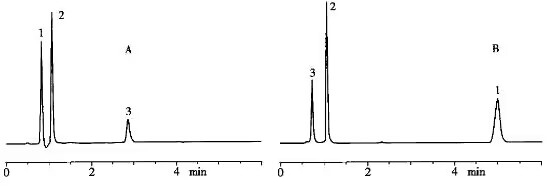
Figure 2 Effect of mobile phase composition on drug retention behavior
2, Calculating the content of HPLC quantification methods can be divided into internal and external standard methods. At the early stage of instrument development, the repeatability of the injection was poor. To ensure the accuracy of the measurement results, the standard internal method was used more often. With the development of automatic injection devices, the repeatability of the injection has been sufficiently precise. Since the 1990s, most internal standard methods have been replaced by external ones.
However, there are still a certain number of HPLC content determination methods using internal standards in pharmacopoeial preparation varieties. Apart from the fact that it has not yet been revised, the previous concern about the reproducibility of the injection is no longer a reason for using the standard internal method, mainly because of the influence of sample recovery or sample preparation methods in the determination of the content of pharmaceutical preparations, the inability to ensure complete extraction of the sample during extraction, centrifugal treatment or other sample solution preparation of the test solution, and the adsorption of excipients on low content drugs, the use of the standard internal method can avoid the effects of the sample due to The standard internal method can avoid the influence of pre-treatment and injection volume errors on the determination results.
3, External standard method content calculation method notes external standard method content calculation formula: content = m pair * P * A sample * V sample / (m sample * A pair * V pair) * 100%
(1) sample weighing sample size
a. the weighing method used is the addition or subtraction method, which is very delicate. Under normal circumstances, the additive method should be used, with one weighing in place, to obtain an accurate weighing volume.
b. the weighing operation must be careful not to spill the sample when transferring it.
c. If the sample is volatile, when weighing directly, it will inevitably evaporate, partly causing errors; also, if the sample is unstable or unstable in solution, then in effect, it is also affecting the sample volume. Also, the sample should be homogeneous, needless to say, for solids. Still, the liquid sample should be homogeneous and shaken well before weighing.
(2) The content of the control
a. The quality of the control is whether the control has a legitimate source and whether its content value is accurate.
b. It is also vital that the control is preserved as required. In addition, if the control is stored in a refrigerator, when weighing, it should be taken out and put back to room temperature in a desiccator before weighing.
Otherwise, it will likely absorb moisture from the air and affect the content value. Similarly, if the control is highly hygroscopic, prolonged exposure to air will also cause changes to its P.
(3) Sample peak area
a. Suppose the analytical method is unreliable and the sample peak area or retention time is prone to change. In that case, the test results are also very poorly reproducible.
b. suppose the instrument system is contaminated to produce residues, and the peak area is not large. In that case, the error produced by this factor is more obvious.
c. integration is important if the sample peak shape is not good enough or if the separation is not good enough.
(4) The dilution volume of the sample
a. Is the volume of the volumetric flask, pipette, etc., accurate? Generally, the volume of newly purchased volumetric flasks aligns with the requirements. Still, you will inevitably buy inferior products that need to be calibrated.
b. Volumetric flask and pipette specifications. This is very important. Generally, we can pay attention to this problem to do reasonable use.
c. Test habit problem, the volumetric flask can not make it to higher than 40 degrees Celsius environment. Otherwise, the volumetric flask will be deformed.
d. Quantitative operation, this includes the use of volumetric flasks and pipettes, precautions: First, when pipette aspiration, the liquid level should not be too high on the scale, never use the carwash ball to blow
when releasing the liquid, and try to keep the same operating time each time, the viscosity of the solution especially need to do so. Secondly, keep the volumetric flask and pipette vertical when observing the liquid
level.
Many people like to pinch with three fingers at this point, but this is not easy to keep vertical. It is best to use two fingers and pinch gently. Third, note that the smaller the area in contact with the volumetric flask
pipette, etc., the better to avoid heating the solution at body temperature.
e. If the control and the sample are not diluted with the same diluent, the two will differ and be affected by temperature changes. For example, the diluent used is water: methanol = 50:50, the control solution used is
last prepared, and the sample used is newly prepared. Because the water and methanol mixture will be exothermic, its volume will also increase. Please wait until the sample is injected, the sample solution is back
to room temperature, and the volume is reduced. It will cause the detection results to be high.

f. If the room temperature changes before and after, it will also cause errors in the analytical results. For example, the control solution into the sample is the normal working hours, the laboratory opened the air
conditioning, the temperature is 20 ℃, and the sample solution into the sample has been by the end of the day, the laboratory staff will be closed when the air conditioning and the laboratory temperature have been
reduced to 10 ℃, the sample solution concentration will certainly also increase at this time.
g. As controls are relatively valuable, they are generally kept in the laboratory for some time. If the next analysis is not back to room temperature, or at this time, the room temperature and the temperature of the control solution preparation have changed significantly, it will cause errors in the analytical results.
h. Some people like to use their hands to hold the volumetric flask after preparing the sample. Those who observe will find that the liquid level is significantly higher than the scale after a while. Suppose the operator does not pay attention to this. In that case, some samples are fed immediately, while others are queued up for a while and then fed, which will cause errors.
Post time: Jan-19-2023






