A new batch of A brand liquid phase purchased inside a pharmaceutical plant to do a certain project out of the peak is bifurcation, which the engineer tossed many times at home, has not been solved. Next to another S brand of the old liquid phase to do the same project, the peak is no problem.
When everyone was at their wits’ end, someone had a bright idea to connect the tubing from the injector to the column on the old S-liquid phase to the new A-liquid phase, and the failure magically disappeared. Later, we found that the volume of the tubing of the old liquid phase was much larger than that of the new liquid phase. This brings us to a phenomenon completely contrary to our common sense in chromatography. The larger the dead volume is, the better the peak is?
To understand this phenomenon, I think you must first talk about the solvent effect in the liquid phase.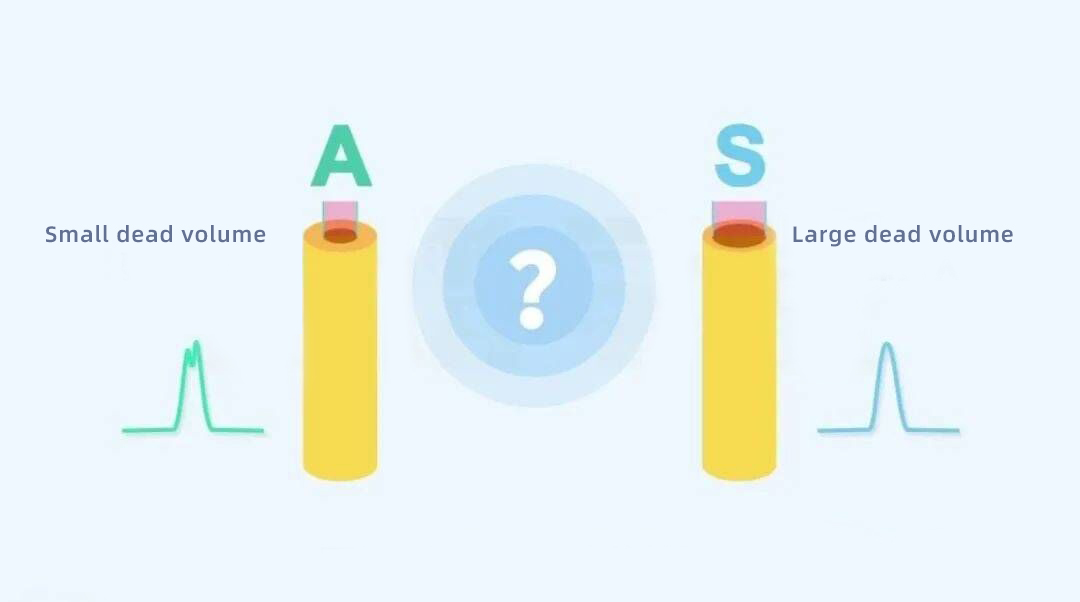
If you have experience in the liquid phase, you may have heard of this name, and you may know that the solvent effect can affect the peak shape of the liquid phase. So what is the solvent effect? How can we avoid the interference of the solvent effect?
The solvent effect, as the name implies, refers to the effect of the solvent used to dissolve the sample on the chromatographic results.
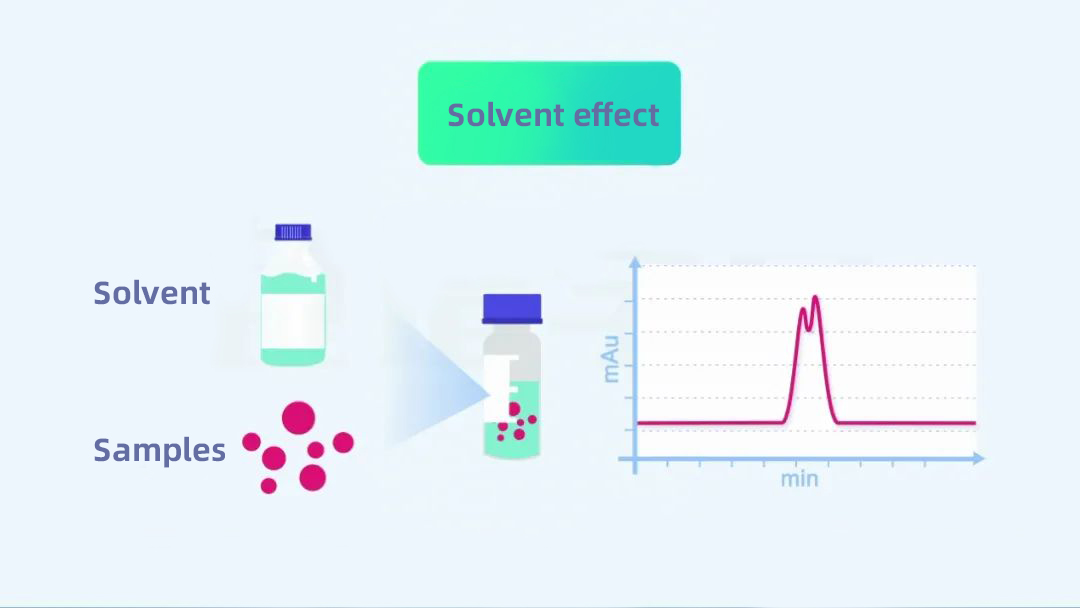
To understand it more deeply, we have to start with how the sample enters the liquid chromatography column. The sample we want to analyze is transferred into the injection vial after a series of pretreatments, dilutions, and volume determinations. The autosampler takes a certain sample volume (e.g. 20 µl) and injects it into the liquid phase system. The sample we are talking about here is the sample molecule we want to analyze and the solvent used to dissolve the diluted sample molecule.
Subsequently, the mobile phase pushes the sample, travels through the line between the injector and the column, and enters the column. The sample solvent is carried away by the mobile phase. In contrast, the sample molecules undergo continuous adsorption and desorption between the stationary and mobile phases inside the column. Finally, they run out of the column into the detector and are detected, forming peaks.
If the sample solvent and the initial mobile phase are the same, then the process is the same as described above, and there will be no problems. However, what if they are not the same? Then the reality is not so simple. If you use a liquid phase method where the initial conditions are 10% acetonitrile-water as the mobile phase, the sample is dissolved in 100% acetonitrile.
The sample enters the liquid phase system and quickly comes to the head of the lc column. Then the sample begins to partition between the stationary phase and the mobile phase, while the sample solvent is carried away by the mobile phase.
Let’s compare the three sample molecules ABC in different positions. The movement of molecule A in the column at the beginning is under the condition of a 10% mobile phase.
And the movement of molecules B and C at the beginning occurs under conditions where the solvent is the mobile phase. And relatively speaking, C has the longest movement time under this condition. 100% acetonitrile has a much stronger elution capacity than 10%, which results in the initial movement of the chromatographic column head. C runs faster, B a little slower, and A the slowest.
This directly leads to the disruption of the uniform distribution of the sample molecules at the beginning of the HPLC column separation, resulting in a variety of peak problems such as precession, off-tailing and splitting at the final peak. We can also see that if the elution strength of the sample solvent is higher than the mobile phase, C will run faster than A, and the solvent effect will be more pronounced.
If the sample volume is larger, the solvent effect will be more obvious because, in this case, the mobile phase will take longer to remove the solvent completely.
So from here, we can also summarize how to avoid or mitigate the solvent effect on the chromatographic peak.
- Try to use the initial mobile phase as the solvent for the sample, or use a solvent with an elution strength close to the initial mobile phase to dissolve the sample.
- However, there are times when pure organic solvents must be used to dissolve certain samples, and we can also use a reduced injection volume to reduce the solvent effect.
- Another trick is to increase the tubing volume between the injector and the column or add a mixer. The goal is to give the mobile phase enough space and time to dilute the sample solvent as much as possible with the mobile phase before the sample enters the column. This way, after the sample enters the column, the sample solvent has been diluted enough not to cause solvent effects.
In the story mentioned at the beginning, the bifurcation of the peaks was solved after replacing a larger pipeline volume for this very reason.
Perhaps at this point, some curious students will ask, “What if I use a solvent with weaker elution capacity than the initial mobile phase? Will the solvent effect also occur?
Besides the different elution strengths, what other factors contribute to the solvent effect? Let’s save these questions and talk to you about them another time.
Post time: Sep-15-2022






