We all know that the packing material of the liquid chromatography column is generally chosen silica gel. Still, there are many requirements for silica gel packing material, summarized as follows.
1, the purity of silica gel
2, types of silica gel
3, the shape of the packing
4, the size of the packing and size distribution
What are the specific requirements for these items? Let me tell you, today, we will talk about the purity of silica gel.
Usually, the purity of silica gel is discussed in terms of the metal ion content, and it is generally recommended that the fewer metal ions in the silica gel packing, the better,
it is because the metal ions on the surface of the silica gel and the silanol base will lead to greater retention of the compound, and this secondary retention effect will lead to the trailing of the peak.
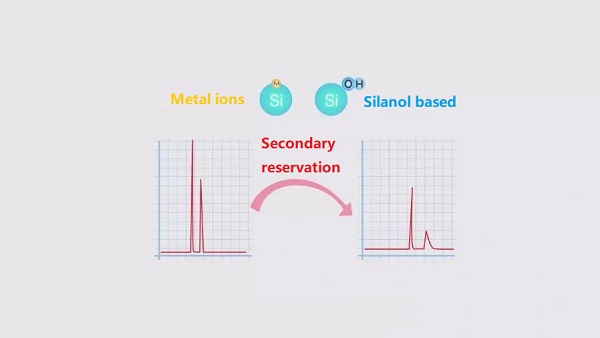
Students may wonder why the presence of metal ions would lead to greater retention of compounds.
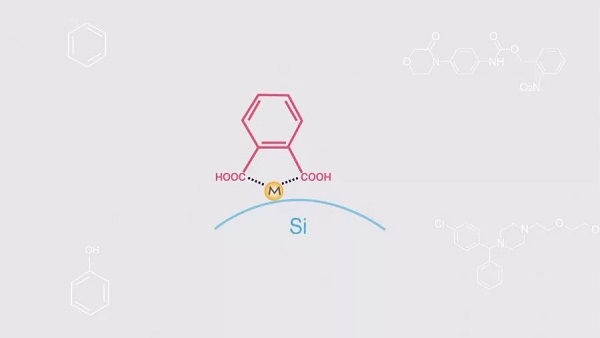
Firstly, metal ions on the surface of silica gel act as chelates, leading to larger retention values for compounds containing multiple polar groups.
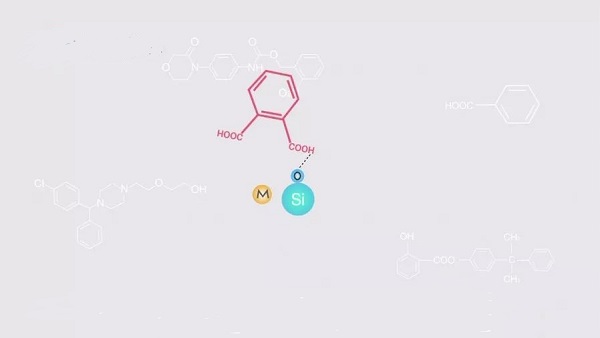
Secondly, the metal ions near the surface activate the silanol groups, making them more likely to interact with acidic or basic compounds. This leads to increased retention values and tailing of peaks.
In the early days, the silica particles used as packing were divided into two types of silica gel: class A and class B. Class A silica gel is negatively charged.
Class A silica gel has negatively charged residual silica hydroxyl groups. It has a high metal content on the biting surface (low pKa for the silica hydroxyl groups), which leads to the tailing of basic compounds.
Type B silica gel is less prone to tailing basic compounds due to its low metal content and high pKa of silica hydroxyl groups.
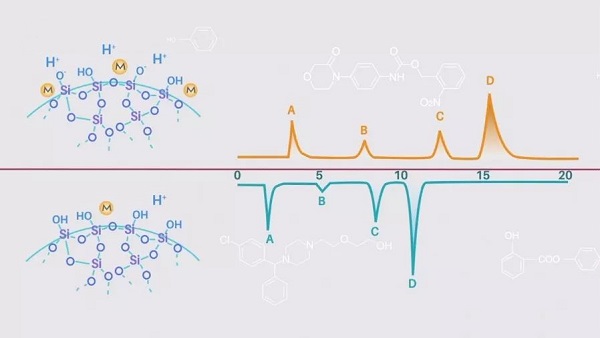
The silica particles used to make stationary phase packing for chromatography columns have all been Type B silica since 1990.
Using Agilent zorbax silica columns as an example, the difference in metal ions between type A and type B silica is as follows:
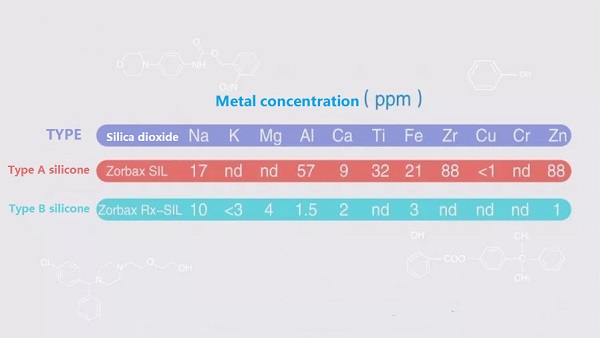
The most effective solution for metal ions on the surface of silica gel is for the supplier to perform an acid wash.
There are cases where up to 1/3 of the metal ion impurities can be removed if the silica gel is treated with a strong acid before chemical modification.
Well, that concludes today’s discussion on the purity of silica gel in liquid chromatography column packing.
Post time: Oct-29-2022






