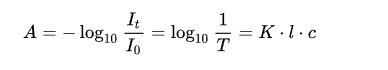In Autumn and winter, it is easy to encounter rain, snow, fog and other weather. We will find that in the fog or heavy rain environment, the same distance under the headlights emitted light than the normal weather transmission rate becomes lower as if the fog or heavy rain absorbs the light.
And the heavier the fog or rain, the lower the transmittance and the more light is absorbed.
This is very similar to the principle of LAMBERT-BEER LAW, on which the UV detector is based.
In the UV detector, we can think of the deuterium lamp as the headlight, the flow cell as the road to drive on, and the liquid sample in the flow cell as the fog or rain. The expression of Lambert’s law is as follows.
In this formula, A is the absorbance, I is the transmitted light intensity, I0 is the incident light intensity, and T is the transmittance. It can also be written as the following equation.
Here b refers to the length of the light range; the longer the length of the light range, the stronger the absorbance. When driving, the farther the distance in foggy and rainy weather, the lower the visibility.
And c refers to the concentration of light-absorbing substances. The higher the concentration of the component to be measured, the greater the absorbance; the greater the fog or heavy rain, the lower the visibility.
ε is called the molar absorbance coefficient, a constant at a fixed wavelength, and the compound’s molecular structure can affect it.
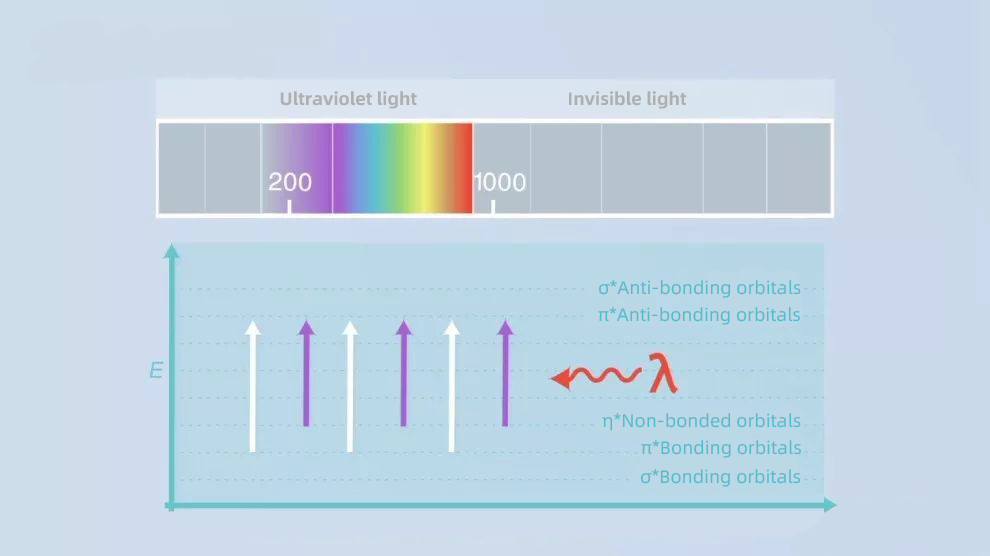
For many organic compounds, the leap of valence electrons in the molecule requires light absorption in the wavelength range of 200-1000 nm.
This happens to fall in the UV-visible region, which is where the UV-visible detector gets its name.
Chromophores
In the structure of organic compounds, most of them are unsaturated groups that can produce UV absorption, and we call these groups to produce UV absorption chromophores. The molar absorbance coefficients of different chromophores are different, so the absorbance of different substances is different, which affects the sensitivity of the substance in the UV detector.
Co-chromophores
In addition, some groups do not produce UV absorption by themselves, but when they combine with these UV-absorbing groups, they will cause the shift of the absorption peak and change the absorption intensity. We call these groups co-chromophores.
This is why some chemical substances have the same chromophore but different UV absorption wavelengths and sensitivities.
The optimal UV absorption wavelength
In addition, since molecules of a substance absorb light differently, we must choose an optimal UV absorption wavelength for the measurement to obtain higher sensitivity. For most students, the selection of the optimal UV absorption wavelength for a compound can be checked through literature or websites…
Or some labs can find the UV absorption wavelength of the right compound if they have a secondary tube array detector by scanning it at full wavelength and then analyzing it with spectroscopy software. However, it is worth noting that: the UV absorption wavelength of the selected compound should avoid the mobile phase cut-off wavelength of about 20 nm to avoid interference in the mobile phase. For example, for the commonly used acetonitrile, which has a cut-off wavelength of 190 nm, the detection wavelength of the compound should be chosen above 210 nm.
For students using liquid chromatography, the UV-VIS detector should be the most commonly used detector, which accounts for about 70% of the usage rate of all liquid phase detectors.
Why can the UV detector be so unique?
1. High sensitivity
First, it can select the maximum absorption wavelength of the sample as the detection wavelength, which can greatly improve the sensitivity. Components with strong UV absorption can reach a minimum detection of 10-12g.
2. Wide linearity range
Second, due to the practical design of the optical path, the UV detector has a wide linear range, and most UV detectors can maintain good linearity at 1.5-2.0 AU absorption.
3. Small temperature effect
Moreover, the temperature has little effect on the UV absorption wavelength and intensity of the compound, so the temperature effect of the detector is small.
4. Small effect of gradient elution
In addition, when the mobile phase has no absorption at the selected sample detection wavelength, the mobile phase is unresponsive, so the gradient elution will also have less effect on the detector’s sensitivity.
5. Non-destructive
In addition, the UV-VIS detector is a non-destructive detector, so that it can be used in combination with preparative chromatography or other detectors. Well, we will introduce the principle of the UV-VIS detector here today, and we will introduce its structure and working precautions in the next course, so stay tuned.
Post time: Sep-17-2022