Protein isolation and purification are important operational techniques widely used in biochemical research applications. There are many protein purification methods, and uHPLCs has summarized the top 9 commonly used methods.
Chromatography was applied to the separation and extraction of phytochromes as early as 1903, where pigments of various colors were arranged in a chromatographic pattern from top to bottom on an adsorbent column, also known as chromatographic separation. 1931 saw the separation of two isomers of carotene using an alumina column, showing the high resolving power of this separation technique, which has attracted widespread attention since then. With the improvement of people’s knowledge and practice of chromatographic techniques and the development of physical-chemical techniques, the scope of application became more extensive. Substances without color could also be separated by this method. All the work at this time was adsorption chromatography using adsorbents. Since the birth of paper chromatography, which applied filter paper as a fixed support in 1944, the development of chromatographic techniques has become more and more rapid. From the 1950s onwards, gas-phase chromatography and high-pressure liquid-phase chromatography appeared one after another. Other methods include thin-layer chromatography, thin-film chromatography, affinity chromatography, and gel chromatography, which also developed rapidly. In biochemistry, chromatography has become a common method for separation and analysis.
Chromatography is a technique built to take advantage of the differences in the physicochemical properties of different substances. All chromatographic systems consist of two phases: a stationary phase, either a solid substance or a component fixed to a solid substance, and a mobile phase, a substance that can flow, such as water and various solvents. When the mixture is separated from the solvent (mobile phase) through the stationary phase, due to the differences in the physical and chemical properties of the components, the ability to interact with the two phases (adsorption, dissolution, binding, etc.) is different, the distribution in the two phases (content comparison) is different, and with the forward movement of the solvent, the components are constantly redistributed in the two phases. The weaker the interaction with the stationary phase, the weaker the component, the smaller the blocking effect when moving with the mobile phase, and the faster the forward movement. Conversely, the stronger the interaction with the stationary phase, the slower the forward movement of the components. Collecting the effluent in parts allows every single component contained in the sample to be obtained, thus separating the components.
Chromatography can be divided according to the chromatographic principle into:
01
Affinity chromatography
Separation is achieved by taking advantage of the specific affinity between the substance to be separated and its specific ligand. When a sample containing mixed components is passed through this stationary phase, only substances with specific affinity to the stationary phase molecules are adsorbed and bound by the stationary phase. Irrelevant components flow out with the mobile phase. The bound affinity can be eluted by changing the mobile phase components. The carrier used in affinity chromatography is called the matrix, and the compound covalently linked to the matrix is called the ligand. The major pairs of biomolecules with specific affinity are antigen and antibody, DNA and complementary DNA or RNA, enzyme and substrate, hormone and receptor, vitamin and specific binding protein, glycoprotein and plant lectin, etc. Affinity chromatography can be used to purify biological macromolecules, the concentration of dilutions, storage of unstable proteins, separation of nucleic acids, etc.
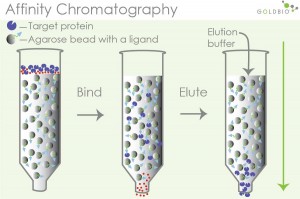
Figure 1. Separation principle of affinity chromatography purification
Features: Affinity chromatography is characterized by high selectivity, high purity, rapidity, and concentration and is mostly used as the first step of crude purification in the separation of recombinant proteins to achieve the removal of the vast majority of impurity proteins.
02
Ion exchange chromatography
A method of separating ionic compounds by using a substance with ion-exchange properties as a stationary phase, which can be reversibly exchanged with ions in the mobile phase. It is mainly used to separate amino acids, peptides, and proteins but also to separate nucleic acids, nucleotides, and other charged biomolecules. The isoelectric point (pI, isoelectric point) characteristics of different proteins make the positive/negative net charge different under different pH buffer conditions. Different ion exchange columns are selected to achieve separation.
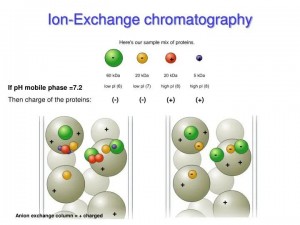
Figure 2. Separation principle of ion exchange chromatography purification
Features: ion exchange chromatography is an adsorptive separation method, and it is reversible, manipulative and achieves sample concentration in the purification process. It is the main technique often used in combination with other methods in purification experiments.
03
Gel filtration chromatography
Also known as molecular sieve filtration or exclusion chromatography, etc., according to the size and shape of the separation, this separation is non-adsorptive. Only a buffer is required throughout the separation process, and the experimental operation is a convenient medical education network to collect and organize the stationary phase in a porous gel. The molecular size of each component is different, and thus the degree of blocking on the gel is also different. The advantages of this method are that the gels used are inert carriers, weak adsorption, mild operating conditions, no organic solvents, and good separation of macromolecular substances. The commonly used gels are the Sephadex G series. Gel chromatography can be used for desalination, separation and purification, determination of the molecular weight of macromolecular substances, the concentration of macromolecular solutions, etc. In the peak spectra, the order of peaks is as follows: large molecules flow out of the chromatographic column first, and small molecules come out later.
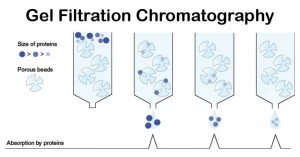
Figure 3. Separation principle of gel filtration chromatography purification
Features: The gels used for chromatography are inert carriers, uncharged, weakly adsorbed, have relatively mild operating conditions, can be performed at a fairly wide range of temperatures, do not require organic solvents, and are unique in their ability to maintain the physicochemical properties of the separated components. It has a good separation effect for polymeric substances.
04
Hydrophobic Interaction Chromatography
A chromatographic method to achieve separation based on hydrophobic differences between biomolecules. Under high ionic strength, the hydrophobic interaction between hydrophobic amino acids in proteins and chromatography medium is enhanced, and the electrostatic force is weakened. During elution, the ionic strength of the mobile phase is reduced to weaken the hydrophobic interaction between the protein molecules and the chromatography medium, and the purification and collection of the target protein are achieved.
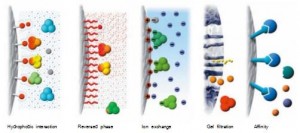
Figure 4. Separation principle of hydrophobic interaction chromatography purification
Features: Hydrophobic interaction chromatography is also an adsorptive separation mode with high selectivity and concentration for target proteins with hydrophobic properties. Hydrophobic interaction chromatography can be used for capture, medium purification, or fine purification stages. Since the sample should be at a higher salt concentration to promote hydrophobic interactions, hydrophobic interaction chromatography is well suited for the capture step after ammonium sulfate precipitation sample precipitation or the intermediate purification step directly after ion exchange chromatography.
05
Reversed-phase chromatography
The separation of biomolecules by reversed-phase chromatography relies on the reversible hydrophobic interaction of the sample molecules and the medium in the eluent. The starting conditions are mainly aqueous and favor a highly ordered water structure surrounding the sample molecules. A small amount of organic modifier, typically 3%-5% acetonitrile, is used to create a “wet” surface. The sample is bound to the filler, and the hydrophobic region exposed to the eluent is minimized.
Separation depends on the equilibrium of the sample molecules in the eluent and on the packing surface. The sample’s distribution depends on the packing’s nature, the sample’s hydrophobicity, and the eluent’s composition (mobile phase), as shown in Figure 5. Initially, the conditions contribute to the extreme equilibrium where 100% of the samples are bound to the column. Since both proteins and peptides have some accessible hydrophobic and hydrophilic amino acids and are relatively large, their interactions with the packing material have a multi-point contact nature.
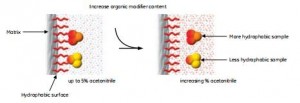
Figure 5. Proteins and peptides bound to reversed-phase chromatography media under aqueous conditions become more hydrophobic after elution
Features: Reversed-phase chromatography can give very high-resolution analytical separations for complex mixtures where viability and quaternary structural recovery are unimportant. Reversed-phase chromatography can purify at high resolution and desalt at low resolution. In the purification step, reversed-phase chromatography is best suited for the fine purification stage when a high-resolution separation of similar components is required when most of the impurities have been removed.
High-Performance Liquid Chromatography (HPLC)
A novel and rapid separation technique developed based on classical liquid phase chromatography and the introduction of the theory of gas phase chromatography. It has the advantages of high separation capacity, high determination sensitivity, can be performed at room temperature, and wide application, etc. It is especially beneficial for separating proteins, nucleic acids, amino acids, alkaloids, steroids, lipids, etc. According to the relative polarity of the mobile phase and stationary phase, HPLC analysis can be divided into two types: normal phase and reversed phase.
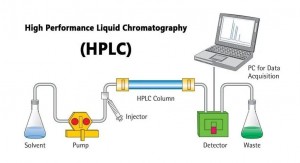
Figure 6. Separation principle of high-performance liquid chromatography purification
Features: High-pressure infusion pump with pressure up to 5000 psi (equivalent to 34 standard atmospheres). A homogeneous stainless steel column is loaded with an ultra-fine support of approximately 3 to 10 microns in diameter. Commonly used supports are small glass beads coated with a layer of 1 to 2-micron silica, which is reacted by thionyl chloride to produce Si-Cl, further connected to hydrophobic alkyl groups such as Si-C18H37, or cationic exchange groups -Si(CH2)n- C6H4SO3H, or anion-exchange group-Si(CH2)nNH2. Such supports can withstand very high stresses and are chemically stable. HPLC with different types of supports can be done by adsorption chromatography, ion exchange chromatography, and gel filtration chromatography. Its analysis is traceable up to 10-10 g level. However, it is used for preparation and can purify samples of up to grams. The spreading time is short, generally taking a few minutes to more than 10 minutes. HPLC is suitable for the analysis and separation of non-volatile and polar substances. HPLC is equipped with a gradient mixer for programmed solvent elution, an integrator and a recorder for data processing, and other electronic systems, making it an advanced analytical instrument that plays an important role in the study of biochemistry, chemistry, medicine, and environmental science.
07
Adsorption chromatography
It refers to the separation of a mixture by the adsorption forces of adsorbents on different substances as the mobile phase passes through the stationary phase. It is one of the earliest chromatographic techniques and is still widely used in separating and preparing various natural compounds and microbial fermentation products.
Adsorption chromatography can be either thin-layer chromatography or column chromatography – the purification method used in protein purification systems. Silica gel is usually used for thin-layer chromatography – silica gel of different particle sizes is made into a paste with water, spread on a glass plate, and then dried at high temperature. Silica gel can also be used for column chromatography. Silica gel is mainly used for separating hydrophobic substances and is not often used in protein chemistry but can be used for separating and analyzing small molecule peptides. Calcium phosphate gel, in contrast to silica gel, is used only for column chromatography, mainly for separating large molecules such as proteins, and presents good results for separating certain enzymes.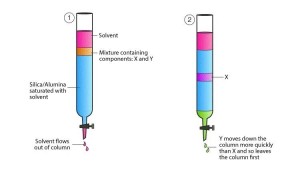
Figure 7. Adsorption chromatography separation principle
Features: Adsorption chromatography is often used to concentrate samples. Some samples with very dilute concentrations are adsorbed on the chromatographic column under specific conditions. Then the conditions are suddenly changed to elute the adsorbed samples from the adsorption column together so that the concentration of the resulting samples can be much higher.
08
Partition chromatography technique
Substances diffuse in two phases at different rates, resulting in different partition coefficients. Partition chromatography is a method that uses the different partition coefficients of each substance to separate the mixture as the mobile phase passes through the stationary phase. The most widely used partition chromatography is partition chromatography on paper with filter paper as the porous support. Next is partition chromatography with porous supports such as silica gel, diatomaceous earth, cellulose powder, starch, and microporous polyethylene powder. There are also normal-pressure liquid-phase partition chromatography and high-pressure liquid-phase partition chromatography that directly partition substances in two immiscible solvents. Partition chromatography is based on the partition coefficient, which can be expressed in the following equation under isothermal and isobaric conditions: K=K2/K1, where K is the partition coefficient; K2 is the concentration of the substance in the stationary phase; K1 is the concentration of the substance in the mobile phase. The partition coefficient is related to the temperature, the nature of the solute, and the solvent, and various substances have different partition coefficients.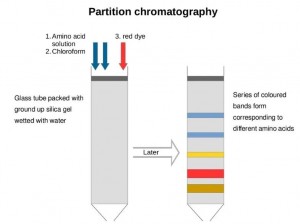
Characteristics: The partition coefficient is the ratio of the concentration of a solute in two immiscible solvents when the dissolution of the solute in the two phases reaches equilibrium. Different substances have different ratios depending on their properties. That is, they have different partition coefficients. Most partition chromatography techniques nowadays are based on a porous substance absorbing a polar solvent, which is always immobilized on the porous support during chromatography and is called the stationary phase. A non-polar solvent that is insoluble in the stationary phase is used to flow through the stationary phase, and this mobile solvent is called the mobile phase. Suppose multiple substances are present between the stationary and mobile phases. In that case, they will be continuously and dynamically partitioned as the mobile phase moves. Since the partition coefficients of the various substances are similar, the distance traveled must be quite long to separate. Conversely, the greater the difference between the partition coefficients of the two substances, the shorter the moving distance when separated. Therefore, the length of the chromatographic column or filter paper used as the stationary phase must be selected according to the actual situation.
09
Chelate Chromatography
The hydrophilic sites in biomolecules can also often form hydrogen and other coordination bonds with each other, and chelate chromatography is based on this principle. In some substrates connected with molecules with coordination ability, such as iminodiacetic acid, such media can chelate zinc, copper, and iron ions, which are not yet saturated with their coordination values. For this reason, the ions can also form coordination bonds with biomolecules such as proteins. The strength of the ligand bonds formed by different biomolecules varies, thus allowing for separation and purification.
Features: This type of chromatography can separate and purify metal-containing proteins such as transferrin and copper salt protein and metal-free proteins.
Conclusion
The process of protein purification is influenced by the physicochemical properties of the protein itself, and the theory and experience of the technician largely determine the success of the purification, so in practice, the appropriate purification method can be chosen according to the experimental requirements and the conditions available in the laboratory.
Post time: Jan-12-2023






