Hydrophilic interaction (Hilic) chromatography appears to be the same as normal phase chromatography in the stationary phase. Can the same column be used for normal phase chromatography and Hilic chromatography? Regarding the mobile phase, which is close to reversed-phase chromatography, what is the difference between the retention behavior of the two modes and the pattern of influence of the mobile phase on retention? Are you also puzzled about Hilic chromatography? Let’s unveil the mystery of hydrophilic chromatography together.
Hilic chromatography sometimes referred to as “aqueous normal phase chromatography” or “reverse phase chromatography”, is simply a polar stationary phase and a polar mobile phase. These compounds are poorly retained in traditional reversed-phase liquid chromatography separations. Modern, highly orthogonal HILIC stationary phases with short equilibration times and extremely high efficiencies help you achieve reproducible, fast HILIC chromatography.
To understand Hilic columns, you must first understand the mobile and stationary phases of Hilic.
1. Mobile phase
In most Hilic separations, the mobile phase is a mixture containing a small amount of water/buffer with the organic phase (typically acetonitrile), with the percentage of water ranging from 3% to 40%.
It is generally believed that the water in the mobile phase of Hilic chromatography will be adsorbed to the surface of the polar stationary phase to form a water film. Then the analytes will be partitioned between the water film and the mobile phase, together with the hydrogen bonding force between the polar functional groups and the stationary phase and the electrostatic force between the ionic functional groups to achieve the retention of the analytes. The role of aqueous membranes is very important. The role of the water film is very important, so the Hilic mobile phase contains at least 3% water. When the proportion of water is greater than 40%, the retention is generally very weak (k≈0).
2. Stationary phase
The stationary phases applied to Hilic chromatography are pure silica gel column, amino column, diol-based column, amide-based column, etc. Pure silica gel column has the advantage that the stationary phase is not easily lost and is most popular when using CAD, ELSD, and LC-MS detectors; the amino column, which is used in Hilic chromatography, is especially suitable for carbohydrate (sugar) separation; diol-based column, which is very hydrophilic, can provide different selectivity.
Hilic compared with normal phase chromatography
1. Difference of stationary phase
The same Silica, NH2, and Diol column, unlike the columns used in normal phase chromatography, the columns designed for Hilic chromatography can be used in the mobile phase of water/organics. In other words, Hilic chromatography requires higher water resistance of the stationary phase. Otherwise, due to the hydrolysis of the stationary phase, there will be a large baseline noise, short column life, and other problems. Therefore, the columns used in normal phase chromatography may not necessarily be used in Hilic chromatography. And the columns suitable for Hilic chromatography can be used in normal phase chromatography.
2. Difference in application scope
Normal phase chromatography is mainly used for fat-soluble compounds with high polarity, while Hilic chromatography is mainly used for water-soluble compounds with high polarity.
3. Summary
Compared with normal-phase chromatography, Silica/NH2/Diol columns used in Hilic chromatography have better water resistance, and Athena NH2-RP has an excellent performance in sugar analysis in Hilic chromatography.
For polar compounds, normal phase chromatography is recommended if the solubility is better in hexane and isopropanol, and Hilic chromatography is recommended if the solubility is better in water.
Hilic compared to reversed-phase chromatography
1. Difference in retention behavior
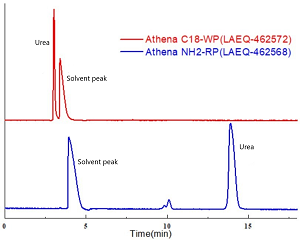
Compared with reversed-phase chromatography, Hilic chromatography has a better retention ability for water-soluble compounds with high polarity. More polar urea is not retained in Athena C18-WP (LAEQ-462572), while it can be well retained in Athena NH2-RP (LAEQ-462568).
2. Elution strength of water in the mobile phase
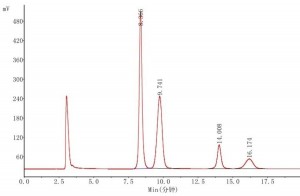
Athena NH2-RP 4.6 × 250mm, 5μm (LAEQ-462568) was used as an example for the analysis of sugar substances.
Mobile phase: acetonitrile/water (75:25)
Chromatographic peaks: 1. fructose 2. glucose 3. sucrose 4. maltose
Application: Honey Chinese Pharmacopoeia 2020 Edition I P374
Mobile phase: acetonitrile/water (70:30)
Chromatographic peaks: 1. sucrose 2. lactose
Application: Lactose Chinese Pharmacopoeia 2020, Part IV P690
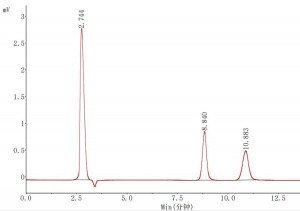
Comparing honey and lactose applications, the peak time of sucrose was shortened from 14.008 min to 8.840 min after changing the mobile phase from acetonitrile/water (75:25) to acetonitrile/water (70:30) using the same Athena NH2-RP 4.6 × 250 mm, 5 μm (LAEQ-462568) column, and the peak of the target compound became earlier when the proportion of aqueous phase in the mobile phase increased. We know that in reversed-phase chromatography, the percentage of the aqueous phase in the mobile phase increases, and the peak of the target compound becomes later. In Hilic chromatography, the aqueous part of the mobile phase behaves as a strong solvent, opposite to the traditional reversed-phase chromatography.
3. Summary
For hydrophilic compounds with high polarity, which are difficult to be retained in reversed-phase chromatography, Hilic chromatography can be used to increase the retention.
In reversed-phase chromatography, the aqueous part of the mobile phase behaves as a weak solvent, while in Hilic chromatography, water behaves as a strong solvent.
Table 1 Comparison of reversed-phase, normal-phase, and Hilic chromatography
|
Separation mode |
Fixed phase |
Mobile phase |
|
RPC |
C18, C8, Phenyl, C4, C30, PFP, CN, …(Non-polar or weakly polar) |
The mix of water, buffered saline solution and methanol, acetonitrile (polar) |
|
NPC |
NH2, Silica, Diol, …(polar) |
Hexane, isopropanol, dichloromethane, ethyl acetate, etc. (non-polar or weakly polar) |
|
HILIC |
NH2-PRr, Silica, Diol, …(polar) |
Mix of water, buffered saline solution and methanol, acetonitrile (polar) |
Hilic chromatography, as a complement to normal and reversed-phase chromatography, uses polar stationery and mobile phases and has great advantages in analyzing water-soluble compounds with strong polarity.
Typically, the proportion of the aqueous phase in the mobile phase is 3%-40%. Too high a proportion of the aqueous phase will result in too weak retention of the target compound on the one hand and accelerate the hydrolysis of the bonded phase on the other.
The hydrolysis resistance of the stationary phase is more demanding, e.g. Athena NH2-RP is particularly suitable for analyzing sugars in Hilic chromatography, with low baseline noise and long column life.
Notes on the use of HILIC chromatographic columns
1. Equilibration of HILIC columns
Equilibrate the new column with 50 times the column volume of 50/50 acetonitrile/aqueous buffer solution (the final concentration of the buffer is 10 mM).
Before starting the sample, equilibrate the column with 20 times the column volume of the starting mobile phase.
For gradient analysis, the column needs to be equilibrated with 10 times the starting mobile phase column volume at the injection interval; inadequate column equilibration will result in retention time drift.
2. HILIC mobile phase needs attention
Always keep at least 5% polar solvent in the mobile phase (e.g. 5% aqueous buffer, 5% methanol, or 3% methanol + 2% aqueous buffer, etc.) to ensure the HILIC silica gel packing is always saturated with water. Keep the percentage of organic solvent (e.g. acetonitrile) in the mobile phase or gradient at least 40%. Do not use phosphate-buffered solution systems as phosphate buffers precipitate in HILIC chromatography mode; using phosphoric acid is not a problem. Aqueous ammonium formate or ammonium acetate buffer systems are more reproducible than aqueous solutions of formic acid or acetic acid. If buffers cannot be used and a mobile phase additive such as formic acid must be used, it is best to use a concentration of 0.2% rather than 0.1%. To get the best peak shape, always keep the concentration of the buffer system at 10 mM in the mobile phase or gradient.
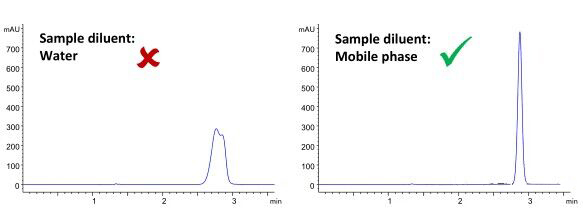
3. HILIC diluents need attention
Try to use 100% acetonitrile to dissolve the sample into the sample. Avoid using water to prepare the sample solution. Choose a weaker HILIC solvent, such as acetonitrile, methanol, isopropanol, etc., to prepare the sample solution. The most commonly used diluent is 75/25 acetonitrile/methanol, a system that adequately balances the sample’s solubility and peak shape. Do not use water or DMSO as diluents, as they will result in poor peak shape. Use the reversed-phase SPE technique to replace water or DMSO with acetonitrile before injecting the sample. If this is impossible, dilute water or DMSO with an organic solvent.
4. Other recommendations for using HILIC start by running a gradient of 95% acetonitrile to 50% acetonitrile
If the sample is not retained, use a mobile phase of 95/3/2 acetonitrile/methanol/water phase buffer solution for isocratic analysis. Replacing water in the mobile phase with methanol, acetone, or isopropanol may also increase the retention of polar compounds. Ensure that the wash/rinse solvent contains the same high percentage of organic phase as the mobile phase. Otherwise, the peak shape will be affected.
5. Column cleaning, regeneration, and preservation
(1) Cleaning and regeneration. A sudden increase in pressure and fluctuations in retention time or separation often indicate that the column is contaminated. The column can be cleaned according to the following operation. Wash with 50/50 acetonitrile/water to remove polar contaminants. If cleaning does not work, clean the column with 5:95 acetonitrile/water. If the system pressure rises above the set pressure limit or if the peaks suddenly diverge, this is usually a sign that the column needs to be replaced.
(2) Storage of the column. If the column is not used for a longer period of time, store the column in 95% acetonitrile; do not store the column in a buffered salt mobile phase, but if the mobile phase contains buffered salt, wash the column with 10 times the column volume of HPLC grade water and then replace it with 95% acetonitrile. Failure to “transition” the column with water may result in salt precipitation when using 95% acetonitrile.
Post time: Dec-01-2022






