I believe that students who use liquid chromatography deal with methanol and acetonitrile daily. But do you know why the mobile phases of liquid chromatography only use methanol and acetonitrile?
Of course, the mobile phase of liquid chromatography also has water and some acids, bases, or buffer salts added to the water. But we will only discuss the organic phase part today and talk about the aqueous phase part next time when we have a chance.
To answer this question, it is necessary to understand the general requirements of liquid chromatography for solvents. Let’s discuss this together, starting from general common sense.
01. Solubility
First, the mobile phase must have a certain solubility for the analyzed sample, right?
This is a very straightforward question. Suppose the sample is not soluble in the mobile phase. In that case, even if you use other solvents to dissolve the sample, as soon as the sample enters the liquid phase system and comes in contact with the mobile phase, it will precipitate and clog the system directly, making it impossible to analyze at all.

Most of the time, it is recommended to use the mobile phase to dissolve and dilute the sample. Suppose the solvent used to dilute the sample is relatively different from the elution strength of the mobile phase. In that case, it may cause the peak dragging phenomenon of the sample, which is called the “solvent effect” of liquid chromatography.
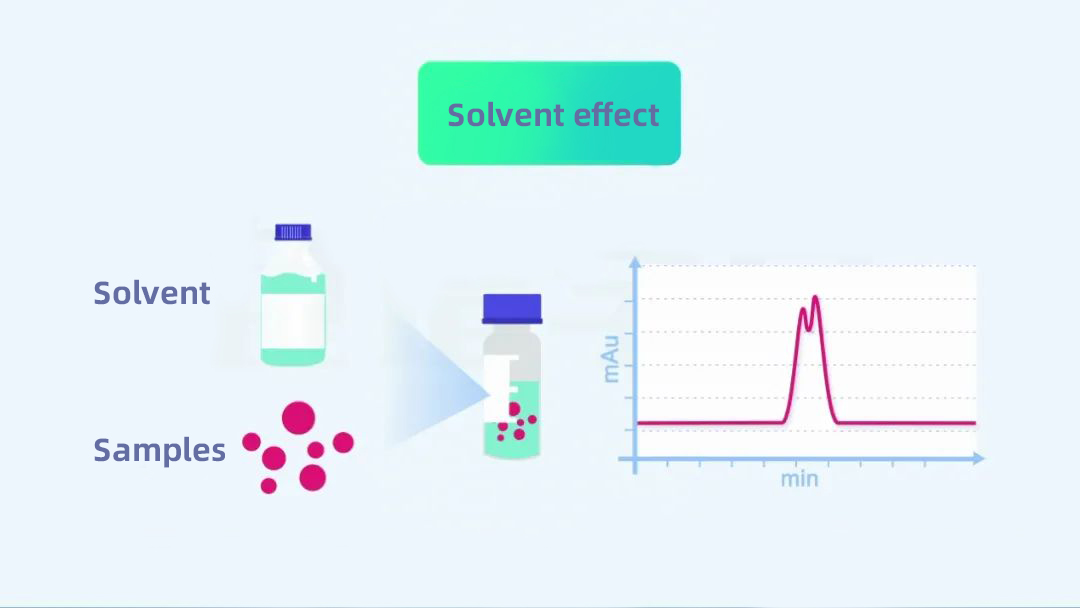
(Click to watch the article “The larger the dead volume, the better the peak?)
02. Corrosiveness
Second, mobile phase solvents should not be corrosive to the liquid phase system.
In most cases, the piping in contact with the mobile phase in the liquid phase system is stainless steel. This is a high specification 316 stainless steel, highly resistant to corrosion. But some solvents, such as chloroform, are very corrosive to stainless steel and may contain peroxide chromatography grade ether, etc.
Sometimes, we also like to use PEEK tubing and fittings because they are easier to install and remove. Although PEEK material has good chemical inertness, it can become brittle and rupture in a long-term environment with solvents such as dimethyl sulfoxide, methylene chloride, and tetrahydrofuran.
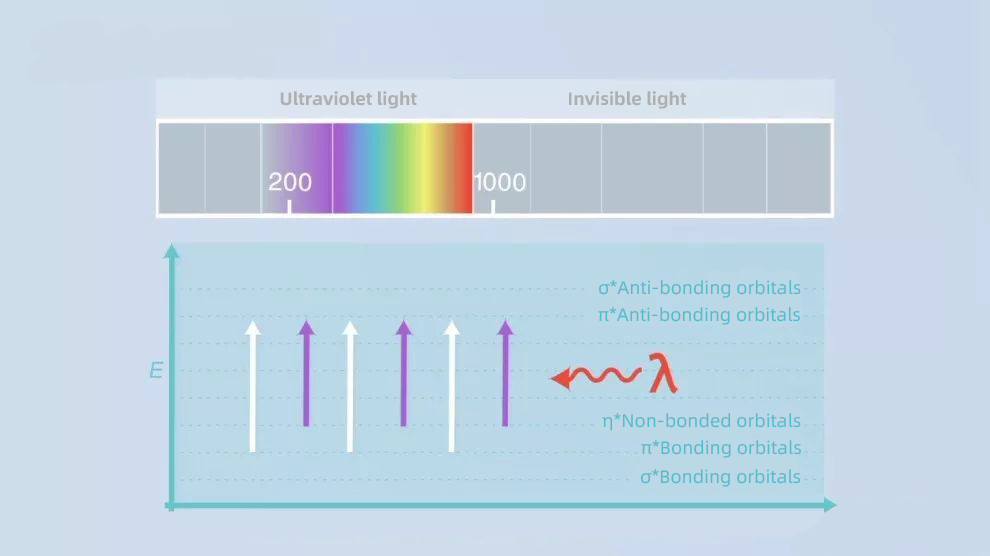
03. UV absorption
In addition, liquid phase mobile phase solvents are supposed to be transparent to UV light.
Because most of the detections in liquid chromatography use a UV detector detect so that there will be no interference with the sample we want to detect.
(Click to view the article “What you need to know about UV-Vis detectors”)
But for assays at lower UV wavelengths than 220 nm, this can be a problem because you will have a hard time finding a completely transparent solvent.
If the mobile phase solvent used at your chosen detection wavelength has some UV absorption, i.e. it is not completely transparent. The specificity of your assay is not as strong, and the linearity of the sample quantification results will be affected.
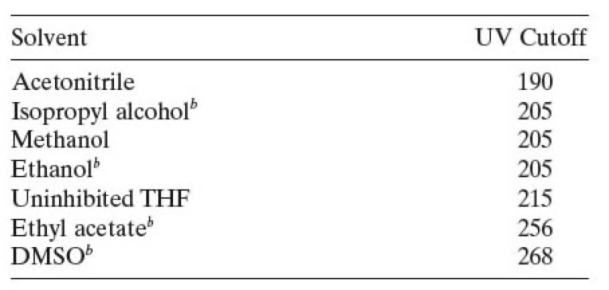
Of course, if you are using gradient elution conditions, the most immediate phenomenon is the drift of the detection baseline.
From this point of view, acetonitrile is superior to methanol because the UV cut-off wavelength of acetonitrile is 190 nm, while methanols are 210 nm. Acetonitrile provides a flatter baseline and better quantitative results at lower wavelengths.
04. Low viscosity
In addition, the mobile phase solvent should be of low viscosity.
If the mobile phase viscosity is higher, the system’s pressure is higher for the same flow rate conditions. In today’s increasing pursuit of analytical speed, the maximum pressure a liquid phase system can provide has been one of the bottlenecks in developing the liquid phase.
Therefore, the lower the viscosity of the mobile phase, the lower the pressure requirement of the system, which is also suitable for fast and efficient liquid phase analysis.
In this respect, acetonitrile is also better than methanol. When methanol is mixed with water, the viscosity becomes higher. Students who have used methanol and water walking through the gradient method can visualize this.
The above requirements of liquid chromatography for mobile phase solvents make methanol and acetonitrile stand out as the most commonly used solvents for liquid chromatography.
Of course, when doing GPC analysis, we also use tetrahydrofuran (THF). For normal phase analysis, Hexane and Cyclohexane are also used. However, regarding the frequency of use and breadth of application, they are far from comparable to methanol and acetonitrile.
05. Non-flammable and low toxicity
Ideally, mobile phase solvents should also be non-flammable and have low toxicity. The first point is from the point of view of laboratory safety, while the latter is from the point of view of health protection for the users of liquid chromatography.

And in this regard, methanol and acetonitrile should be considered less toxic solvents than the more toxic acetonitrile and can be absorbed through the skin. So from the point of view of health protection of operators, methanol has come back to win a game.
Post time: Sep-22-2022






