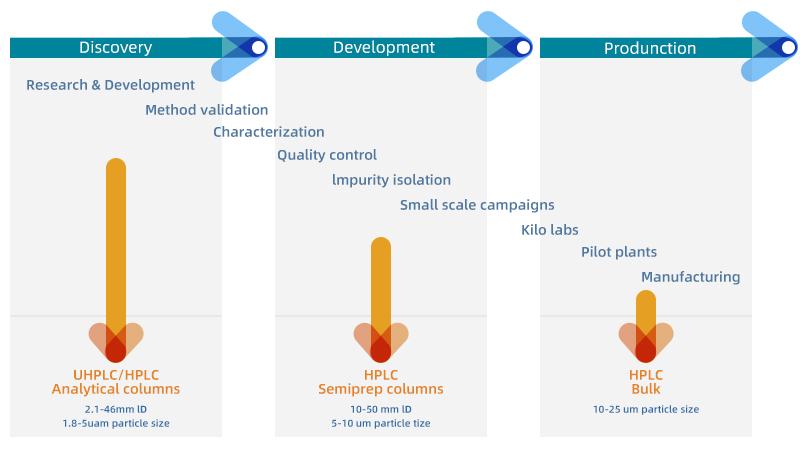
HPLC Columns
Precautions for the use of liquid chromatographic columns:
1) pH range of the hplc columns. C18 reversed phase column are generally suitable for samples in the pH range of 2~8, so the pH of the buffer must be adjusted precisely. The buffer's pH value and the sample's pH value must be adjusted precisely, and the concentration of the buffer must not exceed 0.02 mol/L.
2) Pressure range of the hplc columns. Under normal conditions, the flow rate will gradually increase, and the column pressure will gradually increase until it maintains a more stable range. The maximum pressure should not exceed 4000 Psi, and the column pressure should be recorded every day according to the instruction of each column.
3) Flow rate range of the hplc column. Set the flow rate according to the different columns.
4) The hplc column direction should not be reversed (otherwise, the stationary phase will be damaged).
5) When using buffer, rinse the tubing and column with ultrapure water for 1 hour immediately after sample preparation. Then, rinse with methanol for 40 minutes or more to ensure that the entire tubing and column are clean. Then rinse with methanol for more than 40 minutes to ensure that the whole tubing and column are kept in the organic solvent.
(6) If the instrument is not used for a long time, the column should be taken off and sealed with a plug. Note that the column should not be stored in pure water but in the organic phase (such as methanol, etc.) because pure water is easy to grow mould.
hplc column selection guide
- HPLC Column parameters
Physical Properties:
Column length, inner diameter, such as 250*4.6mm. The general column length is 2-250mm. The longer the column, the higher the separation degree, but the higher the column pressure, the longer the separation time; However, the separation degree is proportional to the square root of the theoretical plate number, so increasing the column length is not the most effective means of separation. In general, 150mm, 5um packing can provide enough plate numbers.
Particle size affects chromatographic separation. The smaller the particle size, the faster the separation and the higher the column efficiency, but the higher the column pressure, the column is easily polluted, resulting in the reduction of column life. Common analytical columns usually use 5um packing, complex multi-component sample separation typically uses 3.5um particle size, and preparative chromatographic columns with larger inner diameters usually use larger particle sizes. If the stationary phase selection is correct, but the separation is not sufficient, choosing a smaller particle size filler is useful. The column efficiency of 3.5-um filler was nearly 30% higher than that of 5um filler under the same condition. However, the back pressure of a 3.5-um column is twice that of a 5um column, so the choice of packing size should be based on the actual situation.
Aperture, 60A, 120A, 300A, etc. The pore size is small, and the porosity is high, the specific surface area is large, the carbon load is high; The pore size of the column packing should match the molecular size to ensure the free entry and exit of the packing pore and the separation and distribution of the bonded phase with the inner surface of the pore. The pore diameter is usually required to be more than 3 times the molecular diameter. Generally, 80-120A is used for small molecules, and 300A is used for large molecules.
The shape of the particles is generally spherical and irregular. When the mobile phase with high viscosity is used, the spherical particles can reduce the column pressure and prolong the hplc column life.
Specific surface area refers to the surface area per gram of packing, such as 180m2/g - 350m2/g, and particle size and porosity; Large specific surface area will increase the reaction between the sample and the bonding phase and increase the retention and separation degree. A small specific surface area can shorten the analysis time and equilibration time. Not a large or small specific surface area is better. We need to choose the appropriate specific surface area.
Chemical properties:
Silicone matrix: the most versatile matrix, strong and easy to chemically modify, but it uses a limited pH range (generally 2-8, special modification can reach 1-12).
Polymer matrix: mostly polystyrene-divinylbenzene or polymethacrylate, chemical stability, wide application of pH range, with stronger hydrophobicity, protein and other samples separation effect is good; However, the strength is small, the organic solvent may cause polymer swelling and damage, the batch repeatability is poor, commercial chromatographic columns are not many, generally expensive.
Carbon load: The proportion of bonded phases on the substrate surface. Suppose the carbon load is high. The retention increases, which is suitable for the analysis of nonpolar compounds.
Bond phase: bond reagents are different, the selectivity of compounds is different, generally long alkyl bond phase (C18 C8) is then short chain (C4 C3) stable; The nonpolar bonding phase is more stable than the polar bonding phase (-NH2).
End Sealing: The exposed silicon HYDROXyl group is bonded and sealed with short chains to reduce the residual silanol group and the chromatographic peak tailing phenomenon caused by the reaction between the tested components, the acidic silicon hydroxyl group. Especially for polar samples, the separation effect of the uncapped column is poor.
Normal phase and reverse phase chromatography:
Currently, the market is mainly based on reverse-phase chromatography, accounting for about 80% of the proportion.
|
Stationary phase polarity |
Polarity of mobile phase |
The peak order |
|
|
Reversed-phase adsorption chromatography |
nonpolar |
Strong polarity (water, methanol, acetonitrile, etc.) |
Strongly polar substances peak first |
|
Normal phase adsorption chromatography |
polarity |
Weak polarity (hexane, heptane, etc.) |
Weakly polar substances peak first |
- Selection of column length and inner diameter:
Length selection: the longer the column, the higher the total column efficiency (n value). The longer the column, the longer the analysis time. 250-300 mm is the most common column length. More than half of the work in the laboratory is used in this specification column, generally used to separate l0 to 50 components of moderate to complex mixtures; Applications requiring higher resolution, 500 -- 600mm, are generally used for separation of more than 50 components or complex samples containing difficult-to-separate substances by temperature-programmed analysis.
Inner diameter: Column efficiency is inversely proportional to the square of the column radius. The smaller the inner diameter, the higher the column efficiency, but the larger the inner diameter, the greater the column capacity, and the more sample injection is allowed. When the injection volume exceeds the column capacity, the true equilibrium cannot be established in each theoretical plate of the column, which will lead to the distortion of the chromatograph bee, the reduction of column resolution, and poor reproducibility. Therefore, small inner diameter columns must be used for complex samples requiring accurate separation. On the other hand, if there are compounds with very different concentrations in the sample, it is necessary to use a column with a large inner diameter to increase the sample capacity. The most common column diameter used in the laboratory is generally 4.6mm.
General selection principles: large pore size column for an analysis of large molecular weight compounds; For high pH or alkaline compounds, high capped or special capped columns should be selected to improve peak shape and prolong column service life.
HPLC Applications:
In terms of environmental protection, HPLC can be applied to
1) Pesticide residue analysis: chlorine-containing pesticides, organophosphorus pesticides, pyrethroids, etc.
2) Carcinogenic substances: nitrofuran and its metabolites, camptothecin, nitrosamines, benzo(a)pyrene, etc.
(3) water quality analysis: endocrine pollutants, etc.
4) Other
......
In biochemical aspects, HPLC can be applied to
1)Protein analysis
2) Peptide analysis
3) Nucleic acid
4) amino acids
5) Other
......